Shri D. Sridhar Babu, Minister for IT and Industries, Government of Telangana, and Rashmi Pimpale, CEO, Research and Innovation Circle of Hyderabad, outline structured clinical and industry immersion programme at BioAsia 2026
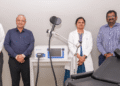
Hyderabad, February 18, 2026: Research and Innovation Circle of Hyderabad introduced the Telangana Hospital and Industry Innovation Bridge at BioAsia 2026, bringing hospitals and industry stakeholders onto a shared platform aimed at strengthening coordination between clinical practice and medical technology development. The initiative was formally introduced by Shri D. Sridhar Babu, Minister for IT and Industries, Government of Telangana, in the presence of senior healthcare representatives.
Participating institutions include AIG Hospitals, Kamineni Hospitals, Bridge Gap Hospitals, Manaha Clinic, LVPEI, Ozone Hospitals, Grace Cancer Foundation and Excell Hospitals. The programme enables structured interaction between clinicians, startups, researchers and manufacturing partners.
Shri D. Sridhar Babu said, “Telangana has consistently focused on building a strong life sciences and healthcare ecosystem driven by innovation and collaboration. The Telangana Hospital and Industry Innovation Bridge integrates clinical insight with industrial capability so that solutions emerging from our ecosystem remain relevant and scalable. As we move towards Vision 2047, this initiative will support long term healthcare growth.”
Shri Sanjay Kumar, IAS, Special Chief Secretary, Government of Telangana, said, “Telangana continues to strengthen connections between healthcare providers, innovators and industry. This initiative supports practical innovation within the life sciences sector.”
The initiative includes clinical exposure visits for startups and researchers to observe hospital workflows and identify unmet needs. Industry visits will give clinicians and medical students access to manufacturing facilities, research centres and regulatory processes. Workshops will cover regulatory pathways, intellectual property processes and validation requirements. Demonstration platforms will allow validated healthcare solutions to be presented to investors and industry stakeholders.
The programme will be implemented in two phases. The first phase will focus on awareness and exposure through immersions and workshops. The second phase will support prototype development, manufacturing readiness, regulatory validation and market access.
Rashmi Pimpale, CEO, Research and Innovation Circle of Hyderabad, said, “The initiative creates direct engagement between clinicians and industry. By encouraging co development and real world exposure, we aim to accelerate the development of clinically relevant healthcare solutions.”
The pilot phase will begin with select institutions before expanding across the state.
At Prittle PrattleNews, featuring you virtuously, we celebrate the commitment and innovation. Led by Editor-in-Chief Smruti Bhalerao, our platform is dedicated to sharing impactful stories that inspire change and create awareness. Follow us on LinkedIn, Instagram, and YouTube for more stories that matter.